
Articles
Learn more about how our products and services help customers just like you on a daily basis.
Flue gas desulfurization (FGD) is a set of technologies used to remove sulfur dioxide (SO2) from flue gases produced from industrial combustion at petrol refineries, chemical manufacturing industries, mineral ore processing plants, and power stations to name a few.
The removal of sulfur dioxide is critical to establishing a safe and clean environment where toxic emissions are kept to a safe low.
Fossil fuels such as coal and oil often contain high amounts of sulfur, and when these fuels are burned around 95% or more of the sulfur is converted to sulfur dioxide (SO2) which is emitted as flue gas.
The main source of sulfur dioxide in the air is a result of industrial activity that processes or uses materials that contain sulfur.
Sulfur dioxide is an acidic gas that reacts easily with other substances to commonly form harmful compounds such as sulfuric acid, sulfate particles, and sulfurous acid. When breathed in, it can irritate the nose, throat and airways with a risk of developing more severe problems over prolonged exposure.
Sulfur dioxide in itself is a major air pollutant which impacts all life. It is also a precursor of acid rain which has significant adverse impacts on forests, freshwaters and soils, in turn killing insect and aquatic life-forms, causing paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and statues.
Due to the damaging nature of sulfur dioxide, stringent environmental regulations have been placed limiting so2 emissions and urging industries to take more action to remove sulfur dioxide from their flue gases.
As sulfur dioxide is an acidic gas it will need to be neutralised before disposal. To do this an alkaline-based sorbent is used to bring the pH level of the sulfur dioxide gas closer to neutral.
Scrubber systems are one of the most efficient ways of reducing sulfur dioxide emissions caused by industrial combustion, and a classic example of an acid-base chemical reaction performed on an industrial scale.
Flue gas desulfurization systems (FGD) or scrubbers are devices capable of sulfur removal efficiencies between 50% to 98%. Typically the highest removal is achieved by wet scrubbers and the lowest by dry scrubbers.
They are used in coal-and-oil fired combustion units including utility and industrial boilers, municipal and medical waste incinerators, petroleum refineries, cement and lime kilns, metal smelters, and sulphuric acid plants.
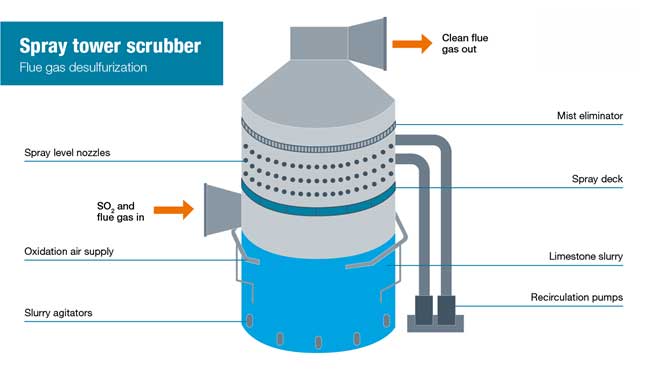
In the wet scrubbing process, flue gas exits the water tube boiler and is drawn into the scrubberspray tower by a fan. The temperature of the flue gas will be in excess of 150-degree Celcius and therefore may be passed through a heat exchanger before entering the spray tower.
The lower third of the spray tower is where the alkaline based slurry is held, this is referred to as the effluent holding tank (EHT). The slurry is most commonly a mixture of ground-up limestone and water. From the bottom of the tower, a heavy-duty centrifugal pump is used to pull through and discharge the slurry from the spray headers nearer the top of the tower.
Often the spray headers are in different levels called a spray deck where the slurry flows along before coming out of the spray nozzles. The alkaline slurry gets sprayed across the entire tray area where the gas meets the slurry. As the sulfur dioxide comes into contact with the slurry it is absorbed by the water and the limestone within the slurry neutralises it.
The gas that continues to rise is now at a pH level closer to neutral, the slurry then drops back down into the EHT. Any remaining slurry that continues to rise is prevented to pass by a mist eliminator. The sulfur dioxide gases are now neutralised and the reaction that has taken place in the slurry has produced calcium sulphite (CaSO3) which in turn can be turned into calcium sulphate (CaSO4) using a process known as forced oxidation.
Oxygen in the form of compressed air is drawn directly into the slurry in the EHT via nozzles from a compressed airline. This bubbles air into the slurry which enables the calcium sulphite to oxidise into calcium sulphate which is also known as gypsum. This step is advantageous as gypsum is able to be sold as a byproduct helping with cost-effectiveness, and it is also easier to separate from the water than calcium sulphite.
The calcium sulphite will gradually settle out to the base of the tank where they can be extracted via a discharge hole. Around 15% of the discharged slurry will contain the suspended solids including gypsum - now referred to as sludge. Once the gypsum is discharged it is sent for dewatering where it can go on to be used as a byproduct for cement and agricultural industries.
The flue gas now exiting the absorber contains very low traces of sulfur dioxide, as little as 2%, significantly reducing the impact that these combustion processes have on the environment.

With the stringent environmental regulations limiting S02 emissions across the world it makes both economical and environmental sense to make flue gas desulfurization a priority in the industrial sector.
Howden provides trusted expertise in turbomachinery used for sulfur, wastewater and other environmental applications. Read more about the world-leading efficient compressors for clean air and clean energy
